August 31, 2009
Researchers in the United States and Spain have discovered that a tool widely used in nanoscale imaging works differently in watery environments, a step toward better using the instrument to study biological molecules and structures.
The researchers demonstrated their new understanding of how the instrument – the atomic force microscope – works in water to show detailed properties of a bacterial membrane and a virus called Phi29, said Arvind Raman, a Purdue professor of mechanical engineering. An atomic force microscope uses a tiny vibrating probe to yield information about materials and surfaces on the scale of nanometers, or billionths of a meter. Because the instrument enables scientists to „see“ objects far smaller than possible using light microscopes, it could be ideal for studying molecules, cell membranes and other biological structures. The best way to study such structures is in their wet, natural environments. However, the researchers have now discovered that in some respects the vibrating probe’s tip behaves the opposite in water as it does in air, said Purdue mechanical engineering doctoral student John Melcher. The probe is caused to oscillate by a vibrating source at its base. However, the tip of the probe oscillates slightly out of synch with the oscillations at the base. This difference in oscillation is referred to as a „phase contrast,“ and the tip is said to be out of phase with the base.
Although these differences in phase contrast reveal information about the composition of the material being studied, data can’t be properly interpreted unless researchers understand precisely how the phase changes in water as well as in air, Raman said.
If the instrument is operating in air, the tip’s phase lags slightly when interacting with a viscous material and advances slightly when scanning over a hard surface. Now researchers have learned the tip operates in the opposite manner when used in water: it lags while passing over a hard object and advances when scanning the gelatinous surface of a biological membrane.
Researchers deposited the membrane and viruses on a sheet of mica. Tests showed the differing properties of the inner and outer sides of the membrane and details about the latticelike protein structure of the membrane. Findings also showed the different properties of the balloonlike head, stiff collar and hollow tail of the Phi29 virus, called a bacteriophage because it infects bacteria.
Original Publication:
Melcher J, Carrasco C, Xu X, Carrascosa JL, Gómez-Herrero J, José de Pablo P, Raman A. (2009): Origins of phase contrast in the atomic force microscope in liquids. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13655-60. Epub 2009 Aug 5.
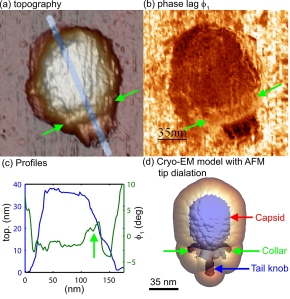
Researchers in the United States and Spain have discovered that an atomic force microscope - a tool widely used in nanoscale imaging - works differently in watery environments, a step toward better using the instrument to study biological molecules and structures. The researchers demonstrated their new understanding of how the instrument works in water to show details of the mechanical properties of a virus called Phi29. The images in "a" and "c" show the topography, and the image in "b" shows the different stiffness properties of the balloonlike head, stiff collar and hollow tail of the Phi29 virus, called a bacteriophage because it infects bacteria. (C. Carrasco-Pulido, P. J. de Pablo, J. Gomez-Herrero, Universidad Autonoma de Madrid, Spain)
http://news.uns.purdue.edu
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: Arvind Raman, atomic force miscorscopy, biological, cells, imaging, John Melcher, Melcher, microscopy, molecules, nanoscale, phase contrast, Purdue, raman, watery |
Research & Development | Verschlagwortet: Arvind Raman, atomic force miscorscopy, biological, cells, imaging, John Melcher, Melcher, microscopy, molecules, nanoscale, phase contrast, Purdue, raman, watery |  Permalink
Permalink
 Veröffentlicht von birgitwashburn
Veröffentlicht von birgitwashburn
August 5, 2009
Scientists from the European Synchrotron Radiation Facility (France) the Forschungszentrum Karlsruhe, the Technische Universität Berlin and the Helmholtz Zentrum Berlin (all Germany) were able to make fast processes inside opaque objects visible, by using white synchrotron radiation to perform hard X-ray radioscopy with high spatio-temporal resolution. The required imaging detector was constructed out of a standard indirect detector in combination with a Photron SA1 CMOS-based camera. Thus, it was possible to investigate pore coalescence and individual cell wall collapse in an expanding liquid metal foam: the rupture of a film and the subsequent merger of two neighbouring bubbles could be recorded with a time sampling rate of 40000 frames per second (25 micorseconds exposure time). The results as published in the Journal of Synchrotron Radiation (http://journals.iucr.org/s/issues/2009/03/00/kv5057/ – open access) allowed to determine that the pore stability in a liquid metal foam is driven by intertia and not the viscosity of the melt. This knowledge is crucial in order to adapt metal foaming process for industrial production.
View videos at:
http://journals.iucr.org/s/issues/2009/03/00/kv5057/kv5057sup1.avi
http://www.alexanderrack.eu/ieee_movie.avi
www.esrf.eu
www.fzk.de
www.tu-berlin.de
www.helmholtz-berlin.de
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: cells, CMOS, coalescence, ESRF, European Synchrotron Radiation Facility, foam, Forschungszentrum Karlsruhe, FZK, Helmholtz, Helmholtz Zentrum Berlin, metal, objects, opaque, Photron SA1, Rack, radiations, radioscopy, recording, resolu, resolutions, spatio-temporal, synchrotron, Technische Universität Berlin, transparent, visualization, x-rays |
Research & Development | Verschlagwortet: cells, CMOS, coalescence, ESRF, European Synchrotron Radiation Facility, foam, Forschungszentrum Karlsruhe, FZK, Helmholtz, Helmholtz Zentrum Berlin, metal, objects, opaque, Photron SA1, Rack, radiations, radioscopy, recording, resolu, resolutions, spatio-temporal, synchrotron, Technische Universität Berlin, transparent, visualization, x-rays |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
Juli 30, 2009
Scientists at Heidelberg University, Germany have developed a new technique for localization microscopy, the “spectral precision distance microscopy” (SPDM). Using visible light, this method allows a single molecule resolution of celullar structures down to the range of few nanometer, about 20 times better than the conventional optical resolution. The researchers invented a new instrument which is a combination of the world’s fastest nano light microscope for 3D cell analysis and the new SPDM technique. Prof. Christoph Cremer of the Kirchhoff Institute of Physics and his team were able to show that SPDM can be realized by common fluorescent dyes, such as the green fluorescent protein (GFP) which can be switched on and off by means of light, as long as certain photophysical conditions are fulfilled. This can be achieved via the so-called “reversible photobleaching” of the dye. So far, only special fluorescent dyes could be used as temporally convertible light signals. According to Cremer there are millions of specimens containing gene constructs with dyes from the GFP group available in biomedical laboratories all over the world. They could be put into immediate use for this new kind of localization microscopy.
www.uni-heidelberg.de
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: 3D, analysis, biomedical, cells, Christoph Cremer, Cremer, dyes, fluorescence, fluorescent dyes, GFP, green, green fluorescent protein, Heidelberg, Kirchhoff Institute of Physics, laboratories, localization, microscopy, photobleaching, photophysical, SPDM, spectral precision distance microscopy |
Research & Development | Verschlagwortet: 3D, analysis, biomedical, cells, Christoph Cremer, Cremer, dyes, fluorescence, fluorescent dyes, GFP, green, green fluorescent protein, Heidelberg, Kirchhoff Institute of Physics, laboratories, localization, microscopy, photobleaching, photophysical, SPDM, spectral precision distance microscopy |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
Juli 23, 2009
Researchers at the University of California, Berkeley, US have developed the CellScope – a new microscope that can be attached to a common mobile phone with a camera to take color images of microorganisms. The CellScope consists of compact microscope lenses fitted in a holder, which is positioned in front of the mobile phones camera. By using an off-the-shelf phone with a 3.2 megapixel camera, the researchers were able to achieve a spatial resolution of 1.2 micrometers. In this way they were able to capture bright field images of Plasmodium falciparum, the parasite that causes malaria in humans and sickle-shaped red blood cells. They were also able to take fluorescent images of Mycobacterium tuberculosis, the bacterium that causes TB in humans. The development of CellScope moves a major step forward in taking clinical microscopy out of specialized laboratories and into field settings for disease screening and diagnoses. „The same regions of the world that lack access to adequate health facilities are, paradoxically, well-served by mobile phone networks,“ said Dan Fletcher, UC Berkeley associate professor of bioengineering and head of the research team. „We can take advantage of these mobile networks to bring low-cost, easy-to-use lab equipment out to more remote settings.“
www.berkeley.edu

CellScope prototype configured for fluorescent imaging (taken by David Breslauer, UC Berkeley)
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: bacteria, Berkeley, bioengineering, blood, Breslauer, bright, bright field, Camera, cell, cell phone, cells, CellScope, clinical, Dan Fletcher, David Breslauer, diagnosis, disease, field, Fletcher, fluorescence, lab, laboratory, light, malaria, Megapixel, micrometer, microscope, microscopy, mobile, mobile phone, Mycobacterium tuberculosis, parasite, phone, Plasmodium falciparum, prototype, research, resolution, screeing, TB, University of California |
Research & Development | Verschlagwortet: bacteria, Berkeley, bioengineering, blood, Breslauer, bright, bright field, Camera, cell, cell phone, cells, CellScope, clinical, Dan Fletcher, David Breslauer, diagnosis, disease, field, Fletcher, fluorescence, lab, laboratory, light, malaria, Megapixel, micrometer, microscope, microscopy, mobile, mobile phone, Mycobacterium tuberculosis, parasite, phone, Plasmodium falciparum, prototype, research, resolution, screeing, TB, University of California |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
Juli 6, 2009
Tomaso Zambelli, a researcher in the group led by Janos Vörös, Professor at the Institute of Biomedical Technology at ETH Zurich, Switzerland, has presented a nanosyringe for automated injection of DNA, RNA and medicines into cells without damaging them.
To create this syringe, called “fluid force microscope”, Zambelli transformed the technology of the atomic force microscope into a microinjection system. In contrast to a conventional manual system, the pressure exerted on the cell by the measuring needle is adjusted so accurately that the cell is not damaged unnecessarily. A laser is responsible for the control, recording every movement of the cantilever and adjusting the force on the cell several thousand times a second. The system also operates under water or in other liquids. To enable the injection of liquids, scientists at the Swiss Center for Electronics and Microtechnology (CSEM) in Neuchâtel installed a microchannel in the cantilever – the diameter of the opening at the needle tip is only 200 nanometers.
In addition to having biological uses, the method could also be applied in the manufacture of microelectronics or microelectromechanical systems (MEMS). The results have been published in Nano Letters.
www.lbb.ethz.ch
www.csem.ch
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: AFM, atomic force microscope, cantilever, cells, CSEM, ETH, ETH Zurich, fluid force microscope, injection, Institute of Biomedical Technology, Janos Vörös, lasers, MEMS, microchannel, microelectromechanical, microelectromechanical systems, microelectronics, microinjection, nano, Nano Letters, nanosyringe, needles, Neuchâtel, Swiss Center for Electronics and Microtechnology, syringe, Tomaso Zambelli, Vörös, Zambelli, Zürich |
Research & Development | Verschlagwortet: AFM, atomic force microscope, cantilever, cells, CSEM, ETH, ETH Zurich, fluid force microscope, injection, Institute of Biomedical Technology, Janos Vörös, lasers, MEMS, microchannel, microelectromechanical, microelectromechanical systems, microelectronics, microinjection, nano, Nano Letters, nanosyringe, needles, Neuchâtel, Swiss Center for Electronics and Microtechnology, syringe, Tomaso Zambelli, Vörös, Zambelli, Zürich |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
Mai 6, 2009
A team of researchers of the University of Georgia (UGA) and the University of California, San Francisco, US has developed a microscope that is capable of live imaging at double the resolution of fluorescence microscopy by using structured illumination. The research was published in Nature Methods on April 26, 2009. “What we’ve done is develop a much faster system that allows you to look at live cells expressing the green fluorescent protein (GFP), which is a very powerful tool for labeling inside the cell,” explained UGA engineer Peter Kner.
www.engineering.uga.edu
 Leave a Comment » |
Leave a Comment » |  Research & Development | Verschlagwortet: cells, duble, fluorescence, fluorescent, Georgia, GFP, green, imaging, Kner, live, live cell imaging, microscopes, microscopy, Peter Kner, protein, resolution, UGA |
Research & Development | Verschlagwortet: cells, duble, fluorescence, fluorescent, Georgia, GFP, green, imaging, Kner, live, live cell imaging, microscopes, microscopy, Peter Kner, protein, resolution, UGA |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
April 30, 2009
During June, 13-25, the 14th annual Living Cell Course will take place at the University of British Columbia Medicine School (UBC) in Vancouver, Canada. This residential course concentrates on all aspects of the 3D microscopy of living cells. Designed for biological research scientists and advanced graduate students, who apply – or plan to – modern 3D imaging, the course want to open up-to-date methods to a wider selection of scientists. The aim of this intense course is to bring students and manufacturers together. The course’s topics include amongst others scanning systems like AODs, mirrors and disks, deconvolution of wide-field and confocal data, dye design, poisson noise QE and S/N, calcium imaging, as well as „how to keep cells alive“.
www.3dcourse.ubc.ca/index.htm

Vancouver, Canada (sorce: pixelio.de)
 Leave a Comment » |
Leave a Comment » |  Education, Events | Verschlagwortet: 3D, AOD, biological, Bristish Columbia, calcium, cells, confocal, course, data, deconvolution, disks, imaging, live, live cell course, living, microscopy, mirr, mirrors, noise, poisson, scanning, UBC, Vancouver, wide-field |
Education, Events | Verschlagwortet: 3D, AOD, biological, Bristish Columbia, calcium, cells, confocal, course, data, deconvolution, disks, imaging, live, live cell course, living, microscopy, mirr, mirrors, noise, poisson, scanning, UBC, Vancouver, wide-field |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
April 28, 2009
PerkinElmer and Accelrys have announced a software collaboration to enable new single cell imaging and analysis techniques. The cooperation is aimed at providing researchers with capabilities for the detection and analysis of single cells via high content screening (HCS) technologies, for faster and better outcomes in identifying cellular markers associated with human health and disease. PerkinElmer’s Columbus software will provide the images containing single cells as well as large amounts of cell level data, and Accelrys‘ Pipeline Pilot platform will furnish intelligent algorithms and image analysis data transfer capability.
www.perkinelmer.com
www.accelrys.com
 Leave a Comment » |
Leave a Comment » |  Collaboration | Verschlagwortet: Accelrys, algorithms, analysis, cells, cellular, Collaboration, columbus, cooperation, imaging, intelligent, PerkinElmer, pilto, pipeline, Pipeline Pilot Platform, platform, relationship, software, technologies |
Collaboration | Verschlagwortet: Accelrys, algorithms, analysis, cells, cellular, Collaboration, columbus, cooperation, imaging, intelligent, PerkinElmer, pilto, pipeline, Pipeline Pilot Platform, platform, relationship, software, technologies |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
April 27, 2009
Asylum Research, in conjunction with IMETUM, Nano Initiative Munich, and Atomic Force F&E, announces the 2nd Euro AFM Forum to be held at the Technical University of Munich (TUM), Garching, Germany, July 1-3, 2009. The event is a conference for AFM researchers to share their cutting-edge research for both materials and life science applications. The forum will combine invited and contributed talks from leading European researchers as well as instructional workshops on AFM equipment. Workshop topics include cell imaging, imaging in liquids, force spectroscopy, electrical characterization and more.
Participants are invited to submit their best AFM image for the Forum Image Contest. An iPod Nano will be awarded for the best image that represents innovative science and has the „cool“ factor. The deadline for submission is June 1, 2009.
www.atomicforce.de/Euro-AFM-Forum-2009.php

Surface of a Chestnut, DC mode in air. Taken by Thomas Gutsmann, Research Center Borstel, Germany (participant of the 2007 event).
 Leave a Comment » |
Leave a Comment » |  Awards, Events | Verschlagwortet: AFM, Asylum, atomic, cells, characterization, contest, cool, cutting-edge, electrical, Euro, Euro Atomic Force Microscopy Forum, force, forum, images, imaging, IMETUM, initiative, life sciences, liquids, materials, microscopy, nano, Nano Initiative Munich, spectroscopy |
Awards, Events | Verschlagwortet: AFM, Asylum, atomic, cells, characterization, contest, cool, cutting-edge, electrical, Euro, Euro Atomic Force Microscopy Forum, force, forum, images, imaging, IMETUM, initiative, life sciences, liquids, materials, microscopy, nano, Nano Initiative Munich, spectroscopy |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi
April 21, 2009
A symposium with a focus on light microscopy and its application in structural biology, organized by the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany will take place form June 22-23, 2009. The symposium aims to bring together structural biologists, cell biologists and light microscopy specialists to explore opportunities and requirements for structural biologists in using different light microscopy techniques and to foster interactions at the interface between structural biology and cell biology.
Planned sessions include:
– Imaging protein-protein interactions
– Protein dynamics
– Correlative light- electron microscopy
– Super-resolution techniques
Deadline for registration is May 3, 2009.
www.embl.org

Heidelberg, Germany (source: pixelio.de)
 Leave a Comment » |
Leave a Comment » |  Events | Verschlagwortet: biology, cells, EMBL, Heidelberg, imaging, interactions, light, light-electron, light-electron microscopy, microscopy, proteins, structural, super resolution, superresolution, symposium, techniques |
Events | Verschlagwortet: biology, cells, EMBL, Heidelberg, imaging, interactions, light, light-electron, light-electron microscopy, microscopy, proteins, structural, super resolution, superresolution, symposium, techniques |  Permalink
Permalink
 Veröffentlicht von aszerdi
Veröffentlicht von aszerdi




 Veröffentlicht von birgitwashburn
Veröffentlicht von birgitwashburn 


